Dec 13 2024
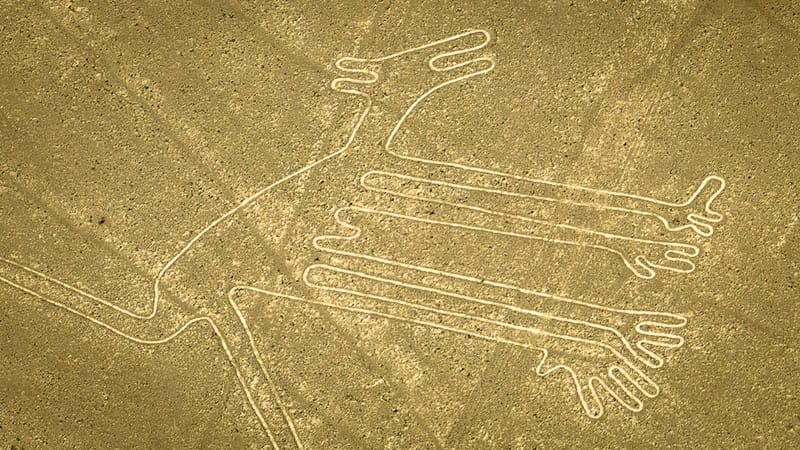
Nazca Lines: Peru’s Geoglyphs
In the arid plains of southern Peru, a series of immense geoglyphs stretch across the desert, forming one of the world’s greatest archaeological mysteries.

Dec 13 2024
In the arid plains of southern Peru, a series of immense geoglyphs stretch across the desert, forming one of the world’s greatest archaeological mysteries.
Dec 12 2024
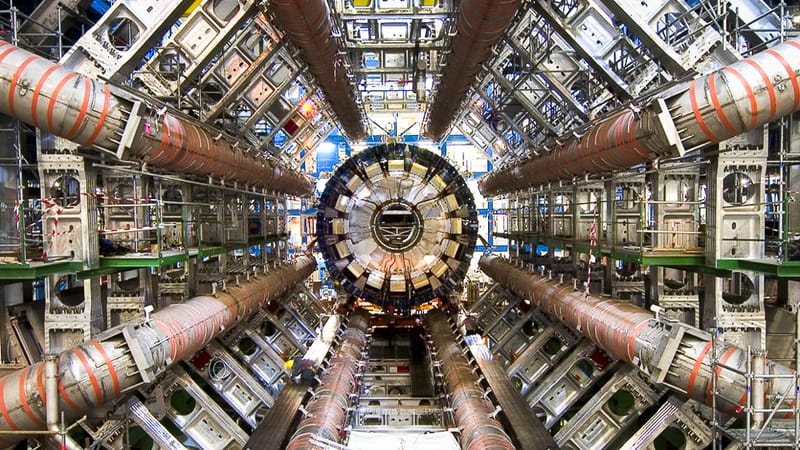
Within its 17-mile circular tunnel, particles travel at 99.9999991% the speed of light, completing 11,245 laps around the collider every second.
The LHC propels particles—usually protons or heavy ions—using a series of superconducting magnets. These magnets, cooled to just 1.9 Kelvin (-271.25°C), guide the particles through the collider’s circular path while powerful electric fields accelerate them to near-light speeds. By the time the particles reach their maximum velocity, they are traveling at over 186,000 miles per second, just shy of light speed.
Reaching this velocity is no small task. As particles accelerate, they gain energy and relativistic mass, requiring even greater force to push them closer to the ultimate speed limit set by the universe: the speed of light. At 99.9999991% of this limit, particles in the LHC achieve incredible momentum, making each collision an event rich with data and discoveries.
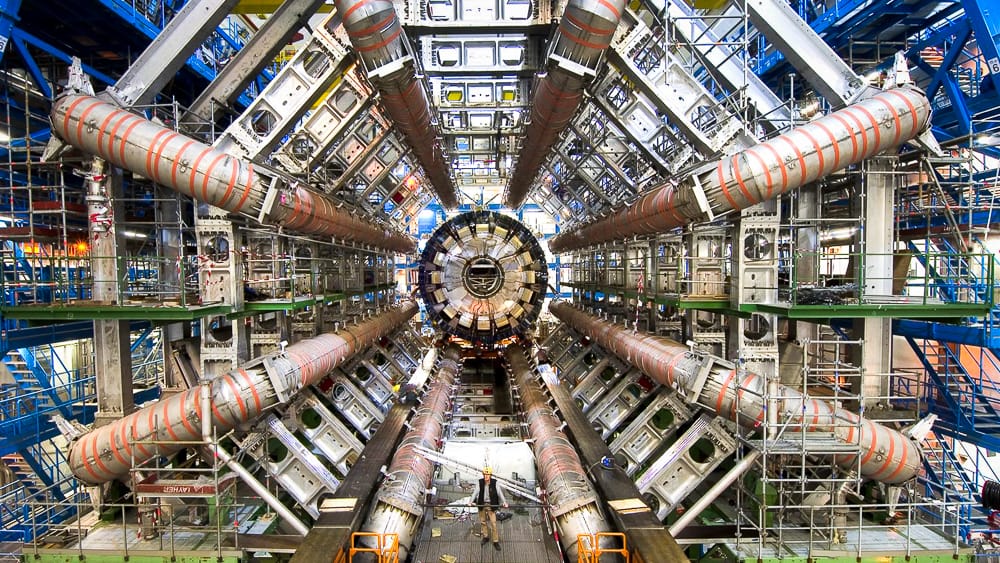
The particles’ 11,245 laps per second are not just an impressive statistic—they’re crucial for the LHC’s mission. This speed allows particles to collide billions of times per second at specific points within the collider. These collisions create conditions similar to those just after the Big Bang, producing exotic particles that exist for only fractions of a second. By studying these fleeting phenomena, scientists unravel the mysteries of the universe, from the nature of dark matter to the origins of mass.
The precision required for these collisions is mind-boggling. The particles, smaller than atoms, must collide head-on despite traveling in opposite directions at near-light speeds. This is akin to firing two needles across the Atlantic Ocean and having them meet precisely in the middle. The success of these collisions has already led to groundbreaking discoveries, such as the detection of the Higgs boson, often referred to as the “God particle.” In this race of light, the particles in the LHC reveal not just the secrets of the cosmos but also the limitless potential of human curiosity.
Learn more at CERN

Nov 20 2024
The patterns captured on the surface of a frozen water bubble reveal the intricate process of crystallization, a natural transformation of water into ice.
When a thin bubble of pure water is exposed to freezing temperatures, it undergoes a rapid yet stunning physical change driven by the unique properties of water molecules. Crystallization begins when the temperature drops below freezing, and the supercooled water within the bubble transitions from a liquid to a solid state.
This transformation starts at nucleation points—tiny imperfections on the bubble’s surface or within the water itself. These points act as seeds where the first ice crystals form. Once nucleation begins, the surrounding water molecules align themselves into an ordered crystalline structure due to hydrogen bonding, a defining feature of water’s molecular behavior.

As the ice crystals grow, they develop intricate, dendritic (tree-like) patterns. This occurs because water freezes anisotropically, meaning that the rate of crystal growth differs depending on the molecular orientation. The feather-like structures arise as the ice expands more rapidly along certain axes, creating the ornate and symmetrical designs visible in the frozen bubble.
The ambient temperature, humidity levels, and even the stillness of the air all influence the formation process. In calm conditions, the crystals grow symmetrically, forming delicate, fern-like patterns. In contrast, slight air movement or fluctuations in temperature can disrupt the process, introducing asymmetries or irregularities.
As the water layer cools rapidly, it freezes before the bubble can collapse. The tension in the bubble’s thin membrane allows it to maintain its spherical shape long enough for the crystallization process to complete, resulting in a frozen globe of intricate ice artistry.
Learn more from our sources: Libbrecht, K. G. (2005). The Physics of Ice Crystals and Petrenko, V. F., & Whitworth, R. W. (1999). Physics of Ice.

Nov 11 2024
As the fourth operational orbiter in NASA's Space Shuttle Program, Atlantis played a crucial role in over 26 years of service.